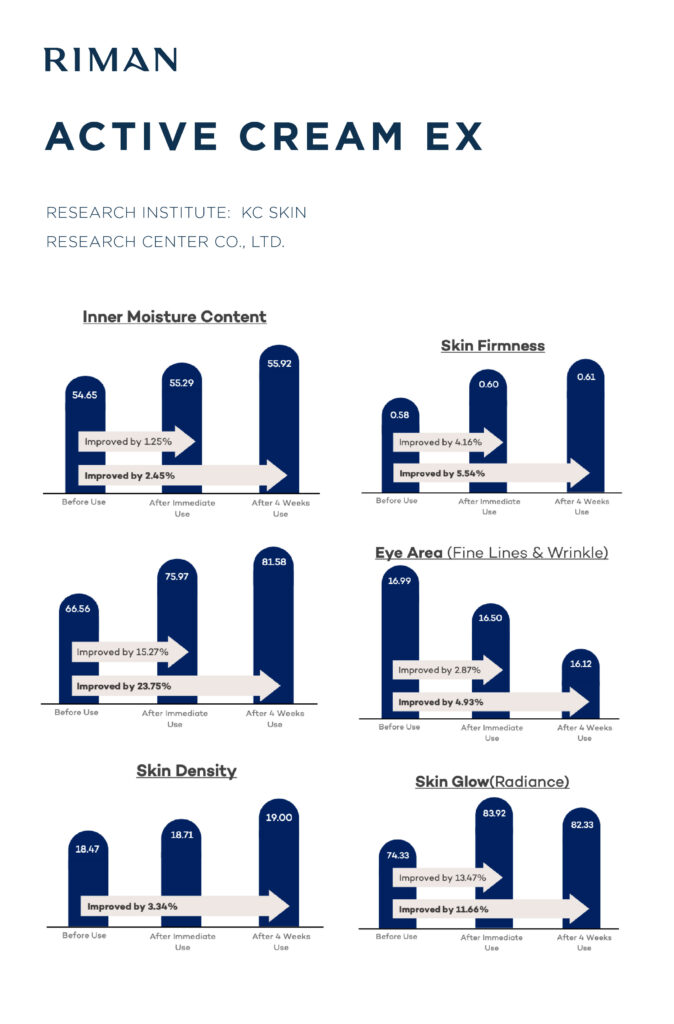
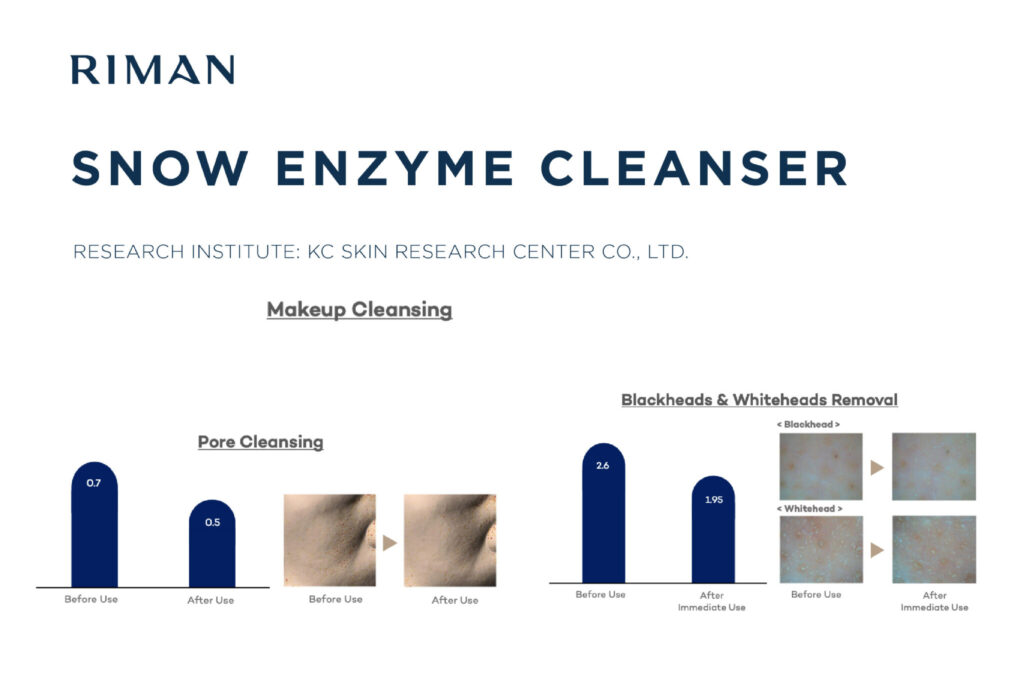
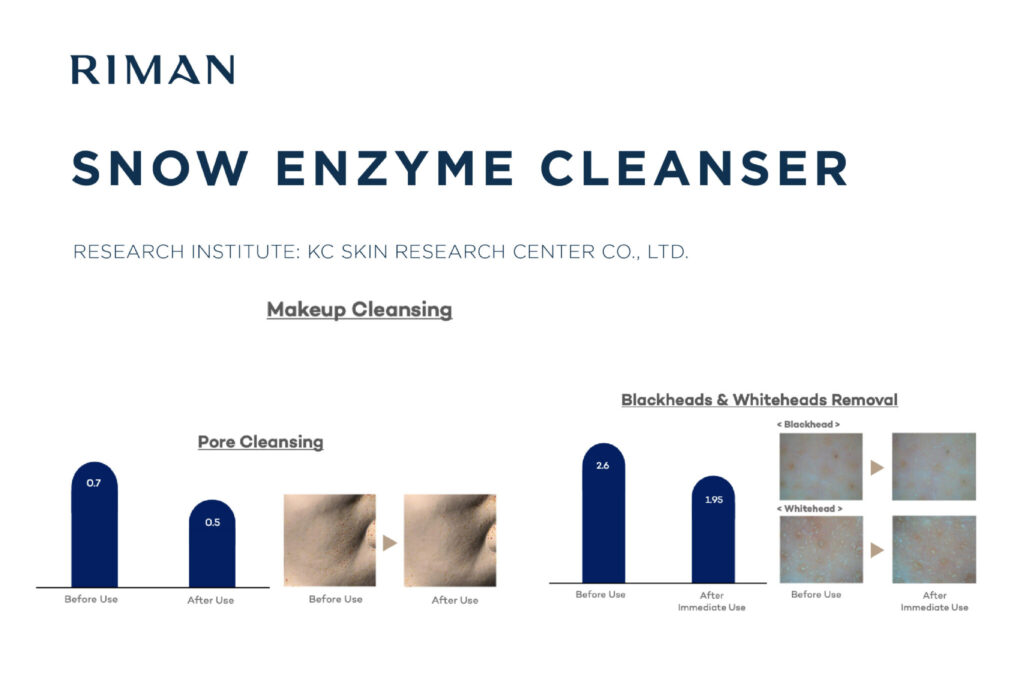
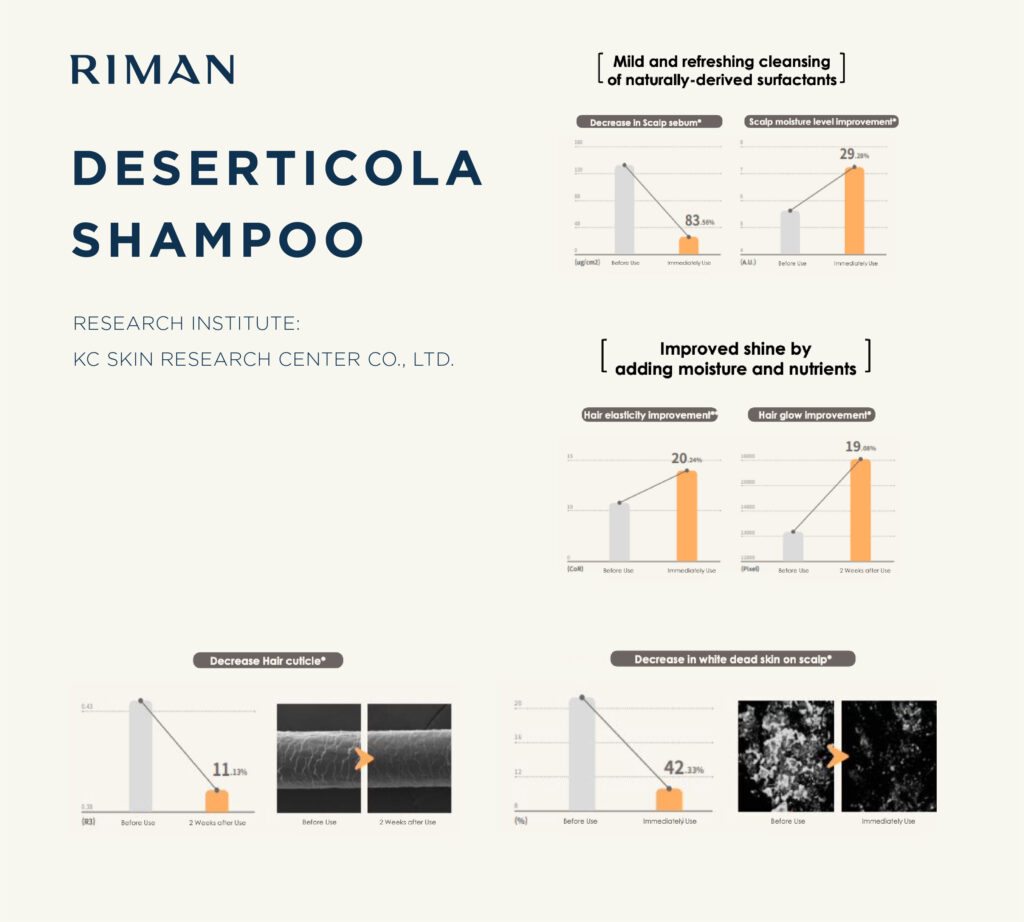
It’s not nice to Entice:
Most people who are involved in the Direct Sales, Multi-Level Marketing industry have heard of “Enticing” or “Cross Recruiting”. While these are technically two different things, we’re going to cover both to make sure you know how RIMAN views both and how to avoid them.
Let’s start with “Enticing”. Also referred to as, “cross line sponsoring, or “unethical sponsoring”, no matter what you call it, its pretty much the same thing. The easiest way to describe Enticing is when a RIMAN Planner tries to recruit an already existing RIMAN Planner to their team. While this can be done in different ways, the bottom line is, if an individual is already enrolled with RIMAN, they are off limits and no attempt to recruit them to a different line or team should be made.
The only way a RIMAN Planner is permitted to change teams is if they submit a letter of resignation and wait 6 months to enroll under a new sponsor; plain and simple, huh?
Now “Cross Recruiting”, also known as “Non Solicitation”, is similar in fashion. This one is just as simple to explain, so here goes: RIMAN Planners may not solicit or attempt to recruit any other RIMAN Planners to ANY direct sale or business opportunity outside of RIMAN. So, if you’ve advised RIMAN that you’re involved with another Direct Sales opportunity, you may not attempt to recruit any RIMAN Planner to join you. If you choose to terminate your RIMAN Planner account, this restriction stays in place for an additional 6 months after you’ve closed your account with RIMAN.
RIMAN takes both issues very seriously, so if you’ve been made aware of an incident, we want to know about it. Reach out to us via email at compliance@riman.com